“The Somni Snore Guard is a unique device. Being simple to prepare and comfortable to use,
it has become a best seller among the serious treatments for snoring.
We frequently receive unsolicited, positive feedback from users.”
British Snoring and Sleep Apnoea Association, (2005)
British Snoring & Sleep Apnoea Association
This oral vestibular shield has been specially designed for those people who snore with their mouth open.
The consequences of mouth breathing at night are often bad breath (halitosis) and snoring, both of which are undesirable and unpleasant for others.
[sp_easyaccordion id=”3612″]
Our Values
Efficiency
Comfort
Safety
Trial Overview
Snoring is a condition that afflicts many people, males more than females and appears to get worse with age (Block et al, 1979). The incidence has been reported to range from 10 – 45 percent in adult populations (Andersson and Brattstrom, 1991; Wagner and Price, 1987; Rice and Persky, 1986). Fujita et al (1981) claimed, that the majority of people over the age of 65 years snore.
Habitual snoring can lead to serious emotional tension between sleeping partners, as well as to the social ostracism of the snorer. The severity of snoring may progressively increased and can lead to obstructive sleep apnoea (OSA), systemic hypertension, cardiac arrhythmias and secondary polyeythaemia (Rice and Persky, 1986).
The aetiology of snoring is multifactorial. The following having been identified as contributory factors:
Loss of tonicity, particularly with age of the tissues of the oropharynx.
Compromisation of the upper respiratory tract due to anatomic abnormalities, trauma or allergic conditions.
- Excess body weight.
- Sleeping on the back.
- Sleeping with mouth open.
- Excessive intake of alcohol prior to sleep.
- Ingestion of large meals shortly prior to sleep.
Factors 1 to 6 can produce partial collapse or occlusion of the oropharyngeal airway while sleeping. In addition, a distended abdomen due to excess body weight or a full stomach exerts pressure on the diaphragm. This reduces the efficiency of the lungs and can lead to laboured breathing. Individually or synergistically the above factors may induce turbulent airflow past the soft pallet, uvula, the posterior region of the tongue and the posterior facial pillars. The resultant vibration of the tissues, particularly during inspiration with an open mouth, produces the audible sound perceived as snoring.
Due to the multifactorial aetiology, Lipman (1990) advocates a comprehensive approach, including a sleep study for the treatment of most snorers. Accordingly, the treatment of choice my range from lifestyle changes to mechanical, medicinal or surgical intervention (Lipman, 1990), Campion (1985) however reported a high rate of success by simply preventing snorers from mouth breathing, using custom fitted vestibular mouth shields.
The aim of this study was to evaluate efficiency of an antisnoring oral vestibular shield that could be fitted to subjects at chair side.
Summary
Stock intra-oral vestibular shields of a heat malleable, semi-flexible ethylene vinyl-acetate material were adapted at chair side to the mouths of 77 chronic snorers. The effectiveness of the shield in controlling snoring was assessed eight to ten weeks after fitting. 77.9 per cent of subject benefited from wearing the device. Of the subjects who failed to benefit, nine (11.7 percent) appeared to have nasal or naso-pharyngeal obstruction. A further 5 (6.5 percent) abandoned the clinical trial due to poor self-motivation, while two subjects were unable to tolerate the shield in the mouth. The potential of the device in the management of obstructive sleep apnoea warrants further investigation.
Results
At the two-week recall, the majority of the subjects were progressing well. Two withdrew from the programme, as they could not tolerate the device, while a further two required adjustment to the shield. A common complaint was loss, or unconscious removal of the shield in the early hours of the morning. Subjects were reassured of the need to allow the oral environment to adapt to the device. A number, who reported difficulty with nasal breathing were advised to use over the counter nasal decongestants.
At the conclusion of the clinical trial, 66.2 percent of the subjects reported complete comfort and complete or near complete elimination of snoring with the shield. A further 11.7 percent found sufficient reduction in the frequency or intensity of their snoring to justify continued use of the device. A substantial number of subjects volunteered, that they had come to regard the shield as a comforter and actually enjoyed sleeping with it.
Two of a total of four subjects, who claimed to suffer from obstructive sleep apnoea reported relief from snoring as from OSA.
6.5 percent found the shield acceptable to wear, but continued to snore through their noses. A further 5.2 percent experienced such difficulty with the enforced nasal breathing, that they would frequently expel the device during sleep. 6.5 percent of subjects were judged to have abandoned the clinical trial due to lack of motivation. One subject continued to breathe and snore through his mouth with the shield comfortably in place.
Discussion
Over 300 anti-snoring devices have been registered at the USA patent office alone (Wagner and Price, 1987). The lack of widespread use, points to the ineffectiveness of these devices. Probably the most difficult aspect of tackling the problem of snoring is securing the co-operation of the snorer. The treatment regime should not be a threat to the snorer and should ideally, improve the quality of life of the sleeping partner and the snorer. If this is not recognized, even an effective treatment approach is likely to be rejected by the snorer. Particular attention must be paid to this, when introducing a device into the sensitive oral environment.
The fact that only 2 of the 77 participants rejected the mouth shield outright speaks well of the acceptability of the device. The group who were judged to have abandoned the trial due to lack of self-motivation did not reject the device on grounds of discomfort. Rather, the impression was gained, that these subjects joined the program merely to placate their sleeping partners and were not genuinely concerned with addressing their snoring problem.
The oral vestibular shield used in this study was based on the device tested by Campion (1985), who moulded a simple, semi-flexible PVC sheet to stone cast of the maxillary and mandibular teeth and vestibule of the subject. Over a period of two years, with the author acting as subject, the shield was modified to its present from. The palatal tag was added to prevent dislodgment of the device during normal nocturnal movements of lips, tongue and mandible. The retention however was not sufficient to prevent expulsion of the shield in the event of difficulties with nasal breathing. The holes in the body of the shield acted primarily to allow air to escape through the mouth during exhalation. Without these, there was a tendency for pressure build-up in the mouth, resulting in the frequent explosive expulsion of the shield. The holes also allowed a degree of inhalation through the mouth, without compromising the effectiveness of the shield. A further feature of the device used in this study, was that the stock shield could be adapted to the individual mouth at chair side.
Campion (1985) reported complete or substantial improvement in the snoring pattern of 80 percent of the subjects he treated. However, of 94 volunteers, he selected and reported on only 55 subjects. The remaining 39 chronic snorers were excluded from his programme for a variety of reasons, such as obesity, alcohol abuse and referral for specialist treatment for nasopharyngeal obstructions (Campion 1985). Thus based on the total number of volunteers, the effectiveness of Campions device translates to 50 percent.
The intention of this study was to determine the proportion of chronic snorers who may benefit from the use of the oral vestibular shield. It was therefore decided, that the sole criterion for participation in the programme would be the ability of the volunteer to breathe through the nose with the mouth closed. It was also felt, that by denying the snorer the opportunity of trying the shield, an unknown number of the volunteers may unnecessarily end up receiving more radical medical or surgical treatment.
The overall success rate of 77.9 percent achieved in this study vindicated the use of the oral vestibular shield as a first option of treatment of snoring. For the five subjects who snored through their noses and the four who found difficulty with nasal breathing while wearing the shield, the device may be considered to have served as a diagnostic tool, indicating nasal or nasopharyngeal obstruction. These volunteers were referred to ear, nose and throat specialists for assessment and treatment.
The present treatment of choice of obstructive sleep apnoea is nasopharyngeal surgery and/or the nocturnal use of a cumbersome and expensive apparatus that provides continuous positive nasal air pressure (Lipman 1990). This study has indicated the need for further investigation of the potential of the oral vestibular shield for the control of OSA.
Materials and Methods
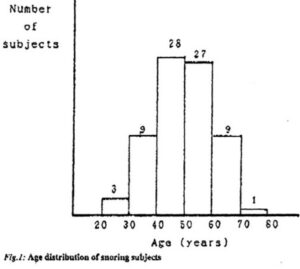
The clinical trials were publicized in a daily newspaper inviting volunteer participants. The first 80 respondents were admitted to the program. One person failed repeatedly to arrive for his appointment while 2 persons withdrew due to ill health, leaving 11 female and 66 male participants. The age distribution of the volunteers is shown in Fig.1, the average age being 49,5 years.

Subjects were counseled in small groups regarding the multi factorial actiology of snoring and informed of the problem that may encounter in adapting to the vestibular shield. In particular, the need to ensure at least one clear nasal passage before retiring to sleep was emphasized. Subjects were also cautioned against unrealistically high expectations and it was stressed, that the oral shield would at best be a means of controlling, rather than curing their snoring. Subjects were interviewed individually and a relevant medical history was recorded. Each subject was required to demonstrate the ability to breathe normally with a closed mouth.
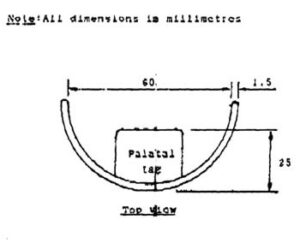
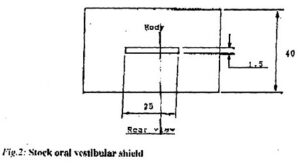
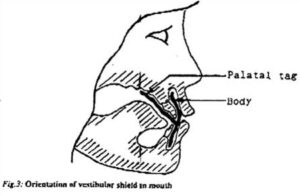
Stock mouth shields, consisting of a curved body and palatal tag (Fig.2) were pre-fabricated from a flexible ethylene vinyl acetate (EVA) material. The stock shield was trimmed to 5mm distal to the second maxillary bicuspid and to 2-3mm short of the limit of the maxillary and mandibular labiobuccal sulci, with the subject in centric occlusion. The shield was placed in water just off the boil (80 – 90ºC) for 10 – 15 seconds. When malleable, the shield was introduced into the subject’s mouth, with the body in the labiobuccal vestibule and the tag clenched lightly between the teeth. The subject was instructed to close the lips and, to simultaneously apply suction and to press the tag against the palate with the tongue for one minute. If necessary, the molding procedure was repeated, until both body and tag were snugly adapted to the subject’s mouth (Fig.3).
Areas of over-extension and interferences with fraena were trimmed with a pair of sharp scissors. Two 2mm diameter holes were punched in the body of the shield in the canine region at the level of the occlusal plane.
Subjects were encouraged to contact the author should they experience difficulties.
The first formal check on progress took place two weeks after the fitting session. The efficacy of the shield was assessed 8 – weeks after the initial fitting. Subjects were questioned regarding the comfort and frequency of use of the shield, while the sleeping partners were asked to report on the effectiveness of the device.
Conclusions
This study has shown, that by wearing an appliance, which prevented mouth breathing during sleep, snoring was controlled in a large proportion of chronic sufferers. The stock oral vestibular shield can be effectively adapted at chair side to the individual and is well tolerated in the mouth. In the vent of possible respiratory distress, the device is readily expelled by the used. The shield could be further viewed as a diagnostic device to identify the snorer who may require more aggressive medical or surgical treatment. The possibility of using the shield for the management of obstructive sleep apnoea warrants further investigation.
Acknowledgements
The author would like to register the contribution of his wife, Alma Veresas observer and co-designer of the oral vestibular shield and to thank his octogenarian for the fabrication of the stock shields. Thanks are also extended to the volunteer snorers for their time and co-operation during the clinical trial.
References
Anderson, L & Brattstrom, V (1991) Cephalometric analysis of permanently snoring patients with and without obstructive sleep apnoea. The International Journal or Oral and Maxillofacial Surgery, 20, 159-162.
Block, AJ, Boysen, PG. Wynne, JW & Hunt, LA (1979) Sleep apnoca, hypapnoen and oxygen desaturation subjects. New England Journal of Medicine, 300, 513-517.
Campion, PCS (1985) The management of snoring: Background and a series of treated cases. Medical Journal Of Australia, 143, 337-338.
Fujita, AS, Conway, W, Zorick, F & Roth, T (1981) Surgical correction of anatomic abnormalities in obstructive sleep apnoea syndrome: Uvulopalate-pharyngoplasty, Otalaryngology – Head and Neck Surgery, 89, 923-024.
Lipman, DS (1990) Stop your husdband from snoring, 1st ed, Ch.8, pp.118-152, Emmanus, Pennsylvania: Rondale Press.
Rice, OH & Persky, M (1986) Snoring: Clinical applications and treatment. Otolaryngoly, Head and Neck Surgery, 95, 28-30.
Wagner, G & Price, RR (1987) An intraoral device to pravent snoring, General Dentistry, May/June, 212-213.
EFFECTIVE ANSWER TO YOUR SNORING PROBLEMS
Why SOMNI snore guard? My story...
As a sufferer of snoring for many years I searched for a simple non-surgical solution to my snoring problem that irritated my wife and affected my marriage.
Eventually after many years and many product failures (pillows, drops, oil rubs) I was considering a surgical solution, besides the cost I was concerned of the permanent effect, and the long term trauma that my body would endure in the operation… It so happened that I had a routine dental appointment a week before the surgery… this was to change my life forever.
You see unbeknown to me my dentist (Dr Veres) had developed the SOMNI Snore guard, once revealing my condition and frustration an intense discussion followed, naturally I had a hesitant approach to a quick fix solution… I simply did not believe that a simple mouth guard would solve my snoring, after all I have been down this road and lost… the persisted plea from the doctor and the constant nagging from my wife encouraged me to prove them wrong!
That night angrily and with great determination to prove them wrong… I followed the instructions and inserted the snore guard and went to bed… What a shocker!
You see every night, all night I would normally toss and turned as the snoring kept me awake, but the morning after using the Somni snore guard I felt great, rested, energized and ready to face the day ahead… but did the snoring stop?
I ran to the kitchen to confront my wife, she was whistling whilst preparing breakfast and yes… she confirmed not a sound! … and added “I told you so”!
I was so impressed; I wanted to share this with the world… I promptly acquired the rights to sell the product online!
We are happy to announce that we have helped thousands of people with their snoring problem; you too can improve the quality of your life forever!
In order to help as many people as possible to overcome their snoring problem we have reduced the pricing… if you’re interested, act quickly, the pricing will revert back to normal shortly!





